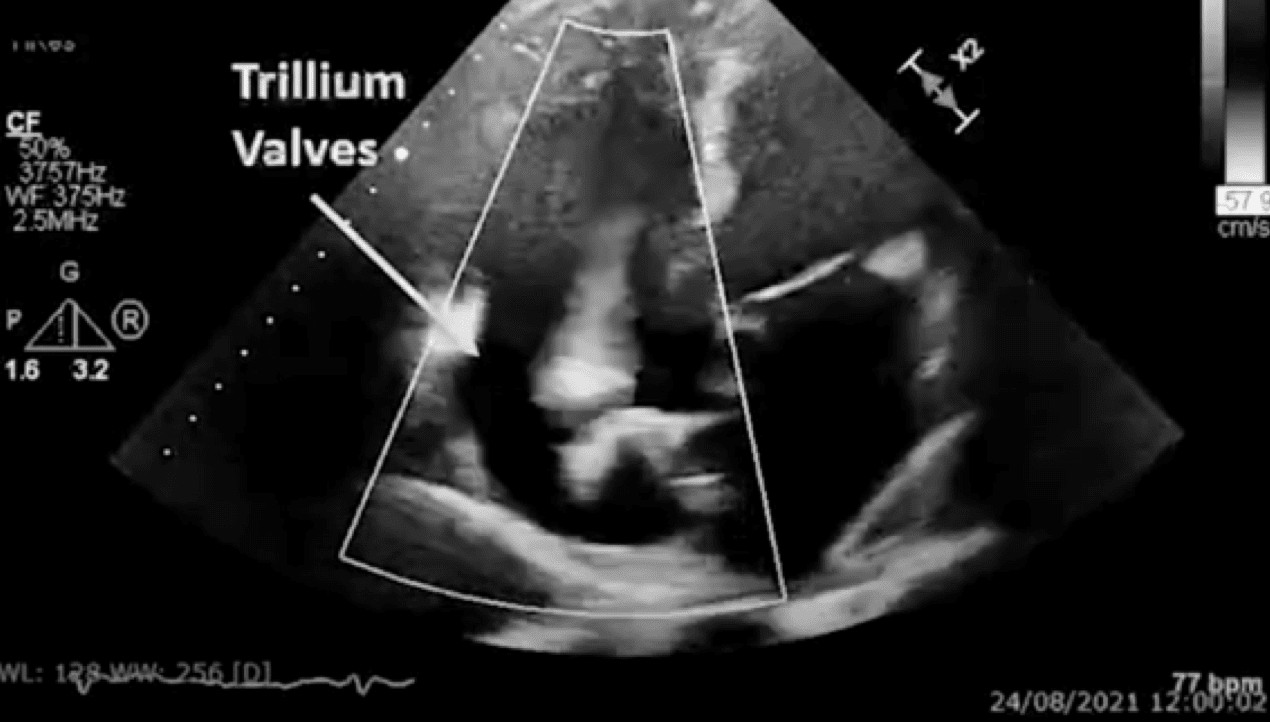
OngoingTrillium™ Early Feasibility Study (EFS)
in the treatment of severe or greater tricuspid regurgitation (TR).
Inclusion Criteria:
- Patient has clinically significant TR graded as severe or greater
- Peak central venous pressure of ≥ 15mmHg
- Patient has NYHA functional classification of III or IV
- Patient is not eligible for standard-of-care surgical or interventional therapy or has refused standard-of-care surgical and interventional therapy or has received standard-of-care TR therapy and remains symptomatic
Exclusion Criteria:
Patients will be excluded from participation if ANY of the
following criteria apply:
- Severe RV dysfunction defined by TAPSE, RVEF, or RVFAC
- Non-suitable anatomy according to CT scan
- Systolic Pulmonary Artery Pressure > 65mmHg
- Moderate or more mitral valve stenosis
- Greater than moderate mitral valve regurgitation or aortic valve stenosis/regurgitation
- Moderate mitral valve regurgitation combined with moderate aortic valve stenosis/regurgitation
- Kidney dysfunction with estimated Glomerular Filtration Rate (eGFR) < 35 ml/min/1.73 m2 within 60 days prior to the index procedure or patient is on chronic dialysis
- Liver cirrhosis or moderate or severe liver disease (Child-Turcotte-Pugh class B or C, or a score of 7 or higher)
- Thrombocytopenia (Platelet count< 100,000/mm3) or thrombocytosis (Platelet count > 750,000/mm3) within 14 days of the index procedure
- In the opinion of the Investigator or the study eligibility committee, the patient’s life expectancy < 12 months
Completed enrollmentTrillium™ First-in-Human (FIH) Study
Trillium™. Twenty (20) patients in 9 investigational sites were enrolled. Patient follow-up was set to baseline,
during the procedure, at discharge, after 1 month, 3 months, 6 months, 1 year, 1.5 years, 2 years, and 3 years
following the index procedure.

OLVZ Aalst
Aalst, Belgium
ZNA
Antwerp, Belgium
Heart and Diabetes Center NRW
Bad Oeynhausen, Germany
Leipzig Heart Center
Leipzig, Germany
Herzzentrum der Charité (DHZC)
Berlin, Germany
Hospital Alvaro Cunqueiro Hospital
Universitario de Vigo
Vigo, Spain
Hospital Universitario de Salamanca
Salamanca, Spain
Hospital Clinic de Barcelona
Barcelona, Spain
Saving Lives While Gaining Clinical Expertise
Our clinical experience extends beyond clinical trials, with compassionate procedures demonstrating our ability to provide quality care even to patients currently deemed untreatable.
With many successful procedures in Belgium, Spain, Germany, Georgia, and Canada, we have improved patients’ lives while showcasing the capabilities of our entire portfolio: Trillium™, Koala™, and Unica™.